Remember Prions? Creutzfeldt-Jakob Disease (CJD)? Chronic Wasting Disease in Deer? Or even Scrapie in sheep? These are all prion diseases. Basically a deformed protein. Not even alive, but capable of great havoc in living bodies.
From NIAID Now | August 23, 2021
Studying infectious diseases in a research laboratory can be like a family building a jigsaw puzzle at home, though with an infinite number of pieces. Dozens of people will enter and exit the research team over many years, making important contributions to the puzzle but never finding the final piece.
In January 2021, Allison Kraus, Ph.D., found the final piece to learning the molecular structure of an infectious prion—a puzzle that her mentor, Byron Caughey, Ph.D., had started 30 years earlier. Together, they and a broad team of scientists at NIAID’s Rocky Mountain Laboratories (RML) in Montana and Case Western Reserve University (CWRU) in Ohio solved the first high-resolution, three-dimensional prion structure. They also obtained unprecedented, but lower resolution, images of another distinct prion strain. Determining prion structures, and their diversity, is fundamental in helping researchers to understand how prion diseases develop, how treatments could be targeted to slow and prevent disease, and how prions compare to proteins that cause related diseases like Alzheimer’s and Parkinson’s.
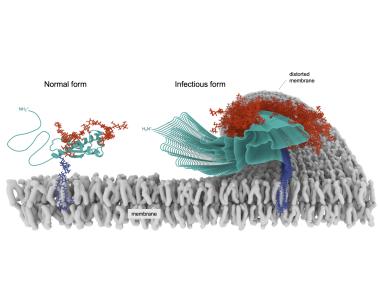
For comparison, the normal form of the prion protein is shown tethered to a cell membrane beside the corrupted form that is infectious. This graphic image was created after scientists analyzed thousands of data points from images collected of the infectious prions.
Credit: CWRU and NIAID
No comments:
Post a Comment